1, Modular design allows flexible configuration, modification, or upgrade, and supports RWT testing (optional).
2, Up to 6 completely independent testing units (motor drive, pressure detection, heating and temperature control, etc.), which are isolated from each other to prevent vibration interference.
3, Test frequency: 5~60Hz (actual frequency depends on the tested valve; a 27mm mitral valve can reach 30-40Hz in actual measurement).
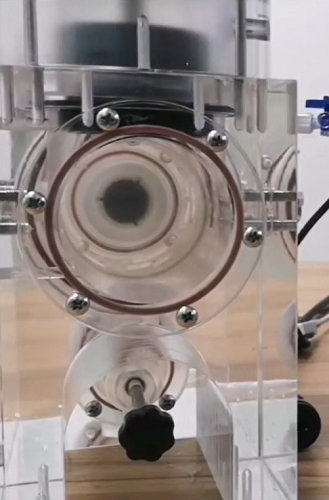
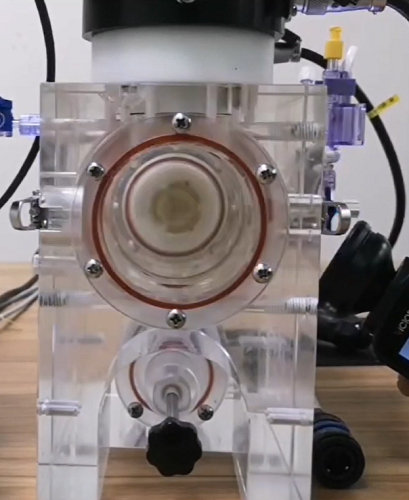
4, Testing units are made of high-transparency acrylic, with observation windows on the front and back, allowing the valve to be observed from all angles.
5, Each unit is equipped with 2 motors (AWT and DFM modes), supporting high-frequency testing of large-size valves.
6, Equipped with 1 high-speed camera at 500FPS, 720*540 pixels (color or black and white).
7, Snap-in design for quick installation and removal of modules and valves.
8, Fast liquid filling and drainage through a water pump and self-sealing quick couplings, no manual filling required.
9, Software uses closed-loop/feedback control, monitoring test conditions of all units/stations in real time and automatically adjusting to ensure operation exactly according to set test conditions while actively preventing excessive or too-low peak pressure differences.
10, Software can simultaneously collect data and images, automatically archive them, and has a built-in player to review images.
ISO 5840-1 Cardiovascular implants – Cardiac valve prostheses – Part 1: General requirements
ISO 5840-2 Cardiovascular implants – Cardiac valve prostheses – Part 2: Surgically implanted heart valve substitutes
ISO 5840-3 Cardiovascular implants – Cardiac valve prostheses – Part 3: Heart valve substitutes implanted by transcatheter techniques
ISO 5910 Cardiovascular implants and extracorporeal systems – Cardiac valve repair devices
If the valve is stented to support the leaflets, the below standard for testing the stent device:
ASTM F2477 Standard Test Methods for in vitro Pulsatile Durability Testing of Vascular Stents
ISO 25539-1 Cardiovascular implants – Endovascular devices – Part 1: Endovascular prostheses
ISO 7198 Cardiovascular implants and extracorporeal systems – Vascular prostheses – Tubular vascular grafts and vascular patches